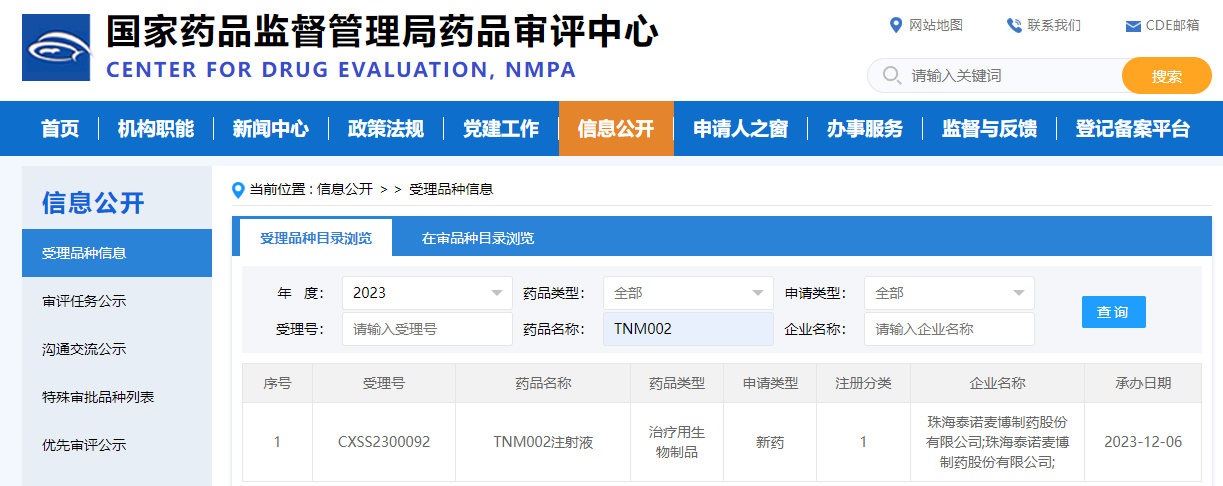
On December 6, Trinomab announced that the New Drug Application (NDA) for TNM002 injection, an innovative monoclonal antibody formulation independently developed by the company, has been formally accepted by China Center for Drug Evaluation of National Medical Products Administration (NMPA)on December 5 and has also been awared with priority review process by the administration on December 6. TNM002 injection is indicated for emergency prevention of tetanus and is a first-in-class anti-tetanus monoclonal antibody product in the field of tetanus prevention in the world.
Passive immunization currently used in clinical practices for the prevention and treatment of tetanus includes equine tetanus antitoxin (TAT) and human tetanus immunoglobulin (HTIG).
According to the position of the World Health Organization (WHO), human tetanus immunoglobulin (HTIG) as passive immunization is currently preferentially recommended for the prevention of post-traumatic tetanus (eg. the wound is contaminated and the victim has not completed the immunization program), which is also essential for the treatment of tetanus cases [1]. In the 1960s, HTIG was developed in Europe and the United States for clinical use, and gradually replaced TAT [2]. In 1991, WHO removed TAT from WHO Model List of Essential Medicines [3].
According to the Frost & Sullivan report, global HTIG productions are mainly from Europe and North America as global blood products companies are located in Europe and the United States. In 2020, more than 25 million doses of HTIG were prescribed in Europe, North America, China, Japan, Southeast Asia and India. Plasma raw materials for immunoglobulin products in Southeast Asia and India are insufficient, and the production capacity is weak. Needs of passive immunization are mainly relied on imported blood products or use of TAT. China has been in short supply of HTIG for years, TAT remains as most often used passive immunization agent for tetanus prophylaxis. In 2022, more than 50 million doses of TAT and only about 10 million doses of HTIG were issued in clinics for tetanus prophylaxis in China.
TAT as an immunoglobulin derived from horse serum, is prone to cause allergic reactions (5% -30% allergic reaction rate). The main clinical manifestations are anaphylactic shock and serum sickness, and "skin test" is needed before clinical use. Desensitization approach has to be applied for patients with positive skin test. During desensitization injection process, 14.1% of the patients still had allergic reactions and 1.2% had anaphylactic shock [4]. Developed countries have banded the clinical use of TAT.
HTIG as plasma-derived products pose significant concerns, which include the risk of transmitting known- and unknown-pathogens despite rigorous testing and the inconsistency in supply of plasma-derived products, leading to worldwide shortages. There is a huge unmet clinical need in the field of passive immunizing agents for tetanus prophylaxis.
TNM002 injection (hereinafter referred as TNM002) is an anti-tetanus toxin native human monoclonal antibody drug developed through Trinomab’s proprietary HitmAb® technology platform. TNM002 is administered by intramuscular injection for emergency prevention of post-traumatic tetanus. TNM002 has great advantages over TAT and HTIG, as reflected in the following four aspects:
√ Good safety: Results of clinical trials show that TNM002 has good safety and tolerability and low immunogenicity. The overall adverse event rate of TNM002 is similar to placebo/HTIG in Phase I-III clinical trials.
√ Good efficacy: Results of the phase III clinical study show that after administration, TNM002 can quickly reach to the levels for tetanus protection, and the titers of anti-tetanus neutralizing antibody are significantly higher and last longer than that of HTIG group. These results indicate that TNM002 injection is superior to the current clinical standard treatment of 250 IU HTIG in the efficacy endpoint.
√ Strong controllability: TNM002 as a recombinant monoclonal antibody product has undergone through rigorously process development and manufactured following rigorous GMP standard. Importantly, there is no use of materials from human or animal sources in the entire process. Thus, there is no potential risk of transmitting blood-borne infectious pathogens in blood products.
√ High accessibility: Compared with tetanus-specific immunoglobulin derived from human plasma products, TNM002 has high potency and is used in small quantity (10mg/dose), while it has high yields (6-7g/L) in production and has short production cycle. Thus, TNM002 can be readily produced in large scale based on clinical needs and market demand. Trinomab has already completed the construction of a commercial production plant and obtained the drug production license for TNM002.
Based on its obvious clinical advantages in terms of safety, effectiveness and accessibility compared with the existing treatment methods, TNM002 has been granted the “Breakthrough” designation from the China NMPA in March 2022, and “Fast-track designation from USA FDA in August of the same year. Based on these designations, accelerated progress of TNM002 NDA can be expected for the review, verification, on-site inspection and other processes and procedures.
Dr. HUAXIN LIAO, co-founder, chairman and CTO of Trinomab, said:
“We are pleased to see NDA for TNM002 has been filed and formally accepted by China NMPA as the positive results of phase III clinical trials for TNM002 have been achieved. TNM002 is the first product reaching to the NDA millstone since the company was founded eight years ago. “This accomplishment shines as an example of the successful synergy between strategic planning and execution as well as the team effort of our company. We will keep close communications with the State Drug Administration of China to promote the process for the expedited approval for TNM002 to revolutionize tetanus prophylaxis and bring new medication options for global tetanus prevention.”
About Trinomab
Zhuhai Trinomab Pharmaceutical is an innovative biopharmaceutical company with a global expansion perspective. The company is mainly engaged in R&D of innovative fully native human mAb drugs. The core technology of the company is known as the fourth-generation antibody technology HitmAb®, a proprietary technology platform featuring differentiated advantages and high efficiency for the discovery of fully native human mAbs as therapeutics against infectious diseases, autoimmune disorders, malignant tumors and other human diseases.
With the mission of “Create Clinical Value” in mind, remarkable progress has been made by Trinomab in recent years. NDA for TNM002 injection (anti-tetanus toxin monoclonal antibody) has been formally accepted early in this month by China NMPA. TNM001 injection (anti-RSV long-acting monoclonal antibody) has entered phase IIb/III clinical trials in later fall of this year. Almost at the same time in this fall, TNM009 injection (anti-NGF monoclonal antibody) and TNM005 injection (anti-VZV monoclonal antibody) have entered in phase I clinical trials in China and the United States, respectively. Clinical trial for TNM006 injection (anti-hCMV monoclonal antibody) will be launched in the first half of the next year as the IND has been approved for TNM006 in 2023. In addition, several more products are in preparation of IND filling with additional more antibody projects on the horizon.
References
[1]http://cdcp.gd.gov.cn/attachment/0/321/321115/3442910.pdf
[2]Luo S D. Human tetanus immunoglobulin and its application [J].Chin J Emerg Med, August 2002, Vol.11, No.4: 285-286.
[3]https://list.essentialmeds.org/recommendations/1000
[4]China Trauma Rescue and Treatment Association,Peking University Trauma Medicine Center ,Chinese expert consensus on tetanus immunization [J]. Chin J Surg, 2018,Vol.56, NO.3: 161-167.