A research article resulted from the collaborative effort by the scientists from Trinomab and academic institutions including the Shanghai Institute of Neuroscience (Chinese Academy of Sciences) and Xi'an Jiaotong University is published in recent issue (May 28, 2025)of the high-impact international journal Cell Reports. Research described in this article identified a cluster of potent neutralizing monoclonal antibodies (mAbs)that are not only capable of neutralize free circulating HCMV, but also able to inhibit cell-cell transmission of HCMV.TNM006 injection being developed by Trinomab was just derived from the best representative (1B03)among this group of mAbs[1]. TNM006 injection has already obtained the approval for clinical trials from the China National Medical Products Administration (NMPA) in May 2023, thereby becoming the first anti-HCMV monoclonal antibody drug to enter the clinical stage in China. The authors in this article further revealed the neutralization epitopes on gB protein of HCMV and mechanisms of action of mAb 1B03 (TNM006) by utilizing cryo-electron microscopy technology[1]. Taken together, this publication provided strong scientific foundation and support for the clinical development of TNM006 injection.
Urgent clinical need: traditional therapies have multiple shortcomings.
HCMV, as one of the eight herpesviruses, is an opportunistic pathogen widely prevalent in humans. The incidence of HCMV infection exceeds 90% in China, with antibody seropositivity observed in 86%-96% of the general population, about 95% of pregnant women and 60%-80% of infants and children [2,3]. Although HCMV establishes lifelong laten infection without clinical symptoms, HCMV infection represents the most common viral complication following immunosuppressive therapy. In clinics, up to 75% of solid organ transplant recipients develop de novo HCMV infection or reactivation of latent virus within three months post-transplantation without prophylaxis, potentially leading to graft failure or mortality. Furthermore, congenital HCMV infection is a leading cause of pediatric sensorineural hearing loss and central nervous system impairments [4].
Current clinical interventions for HCMV prevention and treatment rely primarily on small-molecule drugs and Cytomegalovirus immunoglobulin (CMVIG, not yet marketed in China). Due to the increasing drug resistance, the clinical use of small-molecule agents is severely constrained [5], while CMVIG is limited by plasma supply shortages and potential contamination risks. Therefore, there is an urgent need for safer and more efficacious anti-HCMV monoclonal antibodies to address this unmet medical need.

Address clinical challenges: multifunctional antibodies replace traditional therapies.
Studies have confirmed that in vivo HCMV transmission occurs primarily through direct cell-to-cell spread, not via cell-free virions [6,7]. Consequently, mAbs with the ability not only able to neutralize cell-free viruses but also to inhibit cell-to-cell viral spread are key considerations for identifying suitable antibody candidates. It has been demonstrated that 1B03 (TNM006) is capable of neutralizing cell-free virions with EC50 as low as 0.006 μg/mL and potently inhibiting cell-to-cell spread of HCMV (Figure 1) [1]. HCMV establishes latent infection in most individuals, and viral reactivation during immunosuppression predominantly relies on cell-to-cell spread. Dual anti-CMV biological activities by 1B03 (TNM006) align precisely with this pathogenesis pattern and has advantage over other antibody candidates currently in clinical development [8,9].
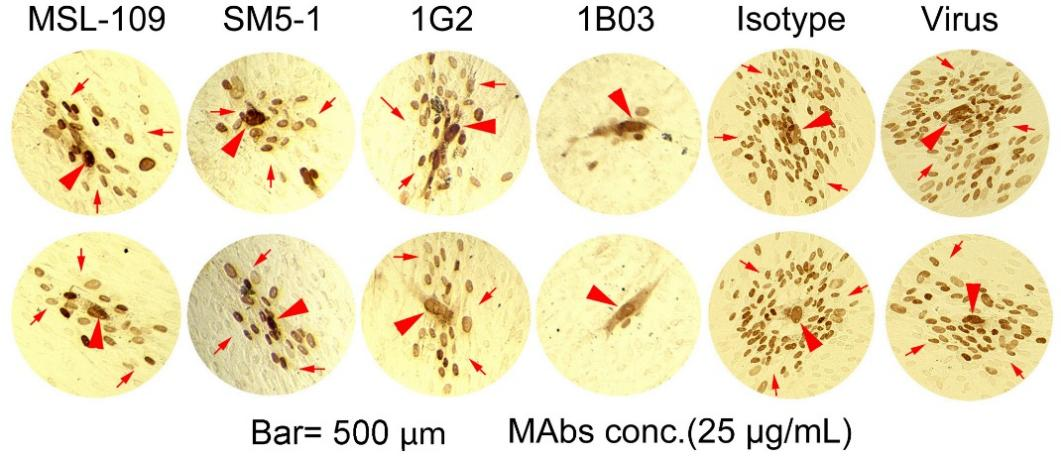
Figure 1. Antibody 1B03 efficiently inhibits the cell-to-cell viral spread. HCMV-infected cells (brown dots) were detected by immunohistochemistry using anti-IE1/IE2 antibody. Smaller brown dots (indicated by arrows) represent IE1/IE2-positive cells as satellite viral infection foci, while larger, darker brown dots (indicated by red arrowheads) show as initial replication centers. Antibody 1B03 nearly completely blocked viral spread from initial replication centers to neighboring cells and outperformed the mAbs (including MSL-109, SM5-1 and 1G2) utilized in clinical trials.
To elucidate the mechanism of action of multifunctional neutralizing antibodies against HCMV, Trinomab collaborated with the Shanghai Institute of Neuroscience (Chinese Academy of Sciences) and Xi'an Jiaotong University on the study to resolve the cryo-EM structure of antibody 1B03 in complex with the envelope gB. 1B03 engages a novel conformational epitope distinct from known gB-targeting antibodies, specifically binding to the critical membrane-fusion subdomain to prevent viral-host cell fusion (Figure 2). The structural insights for 1B03's antiviral potency provide a scientific foundation for novel HCMV therapeutics/prophylaxis and gB-based vaccine design.
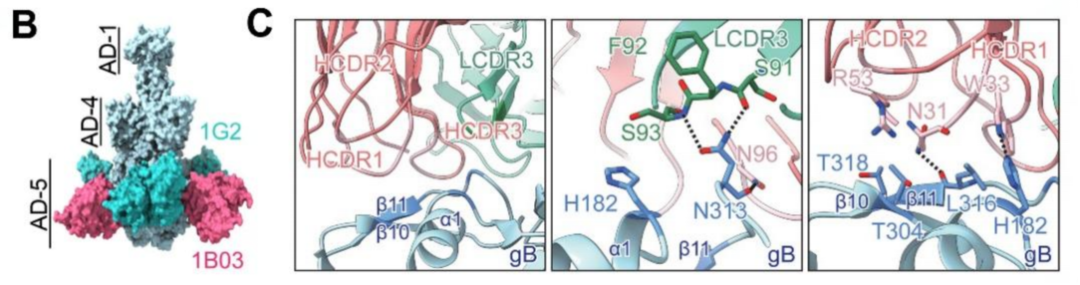
Figure 2. Cryo-EM structure of HCMV gB/1B03 Fab complex. The gB, heavy chain and light chain of 1B03 Fab are depicted in light blue, light coral, and aquamarine, respectively. Key binding residues are highlighted and shown in sticks. Complementary determinization regions (CDRs) of the heavy and light chains are abbreviated as HCDRs or LCDRs, respectively.
This collaborative study, led by Trinomab, in partnership with the Shanghai Institute of Neuroscience (Chinese Academy of Sciences) and Xi'an Jiaotong University, structurally elucidated the mechanism of multifunctional neutralizing monoclonal antibodies against HCMV. These findings provide robust scientific support for development of 1B03 (TNM006) as a therapeutic drug against HCMV.
Drug progress: Fully human antibody TNM006 enters clinical stage
TNM006 injection, developed by Trinomab using the proprietary fourth-generation antibody technology, is a recombinant fully human monoclonal antibody against HCMV via inhibiting gB-mediated membrane fusion. Compared to CMVIG, TNM006 demonstrates superior potency in neutralizing HCMV in vitro.
TNM006 injection is produced through standardized industrial-scale manufacturing processes. Successful development of TNM006 could significantly reduce costs and eliminate the blood-derived dependency and risk of infectious diseases and provide a new option in treatment/prevention of HCMV infection to enhance the success in solid organ transplantation.

References:
[1] Changwen Wu, et al. Structural basis of human cytomegalovirus neutralization by gB AD-5-specific potent antibodies. Cell Rep. 2025,44(5).
[2] Li Z, Tang Y, Tang N, et al. High anti-human cytomegalovirus antibody levels are associated with the progression of essential hypertension and target organ damage in Han Chinese population. PloS one. 2017,12(8):e0181440.
[3] Xiang-lan Liu, Xue-ying Gu. differential diagnostic methods between primary and non-primary cytomegalovirus (CMV) infections. Chinese Journal of Reproductive Health. 2008,19:95-98.
[4] Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007,17(4):253-276.
[5] Erice A. Resistance of human cytomegalovirus to antiviral drugs. Clinical Microbiology Reviews. 1999,12(2):286-297.
[6] Sattentau Q. Avoiding the void: Cell-to-cell spread of human viruses. Nat. Rev. Microbiol. 2008,6:815-826.
[7] Jackson J.W, Sparer T. There Is Always Another Way! Cytomegalovirus’ Multifaceted Dissemination Schemes. Viruses. 2018,10:383.
[8] Pötzsch S, et al. B cell repertoire analysis identifies new antigenic domains on glycoprotein b of human cytomegalovirus which are target of neutralizing antibodies. PLoS Pathog. 2011,7(8):e1002172.
[9] Nina R, et al. Neutralizing antibodies limit cell-associated spread of human cytomegalovirus in epithelial cells and fibroblasts. Viruses. 2022,14(2):284.