The next-generation "tetanus shot" Sintetol (generic name: Siltartoxatug Injection) has officially passed the review by the National Healthcare Security Administration (NHSA) and has been included in China’s National Reimbursement Drug List (NRDL) (2025) ! As a first-in-class recombinant anti-tetanus toxin monoclonal antibody drug originated from China, Siltartoxatug Injection achieved NRDL inclusion within one year of its marketing approval in February 2025. This swift inclusion underscores the high recognition of its clinical value and serves as a tangible reflection of the NHSA’s commitment to “supporting genuine innovation.” This move not only ensures drug availability in clinics but also enables patients to conveniently access high-quality and effective treatment through national healthcare security system.
The updated NRDL was officially released on December 7, 2025, and will take effect uniformly across the country on January 1, 2026.

[Image: Cover of the National Reimbursement Drug List (2025)]
Siltartoxatug Injection is a Class I innovative drug developed by Zhuhai Trinomab Pharmaceuticals Co., Ltd. ("Trinomab"). As an advanced next-generation prophylactic for prevention of tetanus, it is indicated for emergency post-exposure prophylaxis (PEP) of tetanus in adults. Administered as a single intramuscular injection, it acts rapidly and provides long-term protection. It requires no prior skin test, no dose adjustment based on body weight or wound size, and (for outpatients) no mandatory post-injection observation period per the package insert, offering patients a safer, more effective and more economical option for prevention of tetanus in case of tetanus prone wound.
For Patients: Alleviation of Payment Burden
The shift from out-of-pocket payment to the insurance reimbursement program makes reliable tetanus prophylaxis readily accessible. It is noteworthy that approximately 10% of tetanus cases have an incubation period of less than 48 hours, sometimes even under 24 hours, making the prophylactic window extremely short. NRDL coverage enables patients to promptly access this world-leading optimal solution in emergencies, minimizing delays in the optimal prophylactic window due to cost concerns.
lFor Hospitals and Medical Staff: Improvement of Clinical Standards and Optimization of Diagnosis and Treatment Efficiency
Siltartoxatug Injection represents a shift from reliance on blood-derived products to the safer and more consistent recombinant DNA technology. Its NRDL inclusion, coupled with features like “no skin test required” and “single-injection full protection,” is expected to significantly improve emergency department workflows and alleviate pressure on medical resources. It not only reduces medication-related risks and simplifies procedures by eliminating skin testing but also elevates the overall standard of care for tetanus prophylaxis. Its subsequent rapid adoption in primary healthcare institutions will help standardize and raise national tetanus prevention protocols, bridge regional healthcare gaps, and transform this innovation from being merely available to being widely accessible.

Next-Gen "Tetanus Shot" Siltartoxatug Injection versus Traditional "Tetanus Shots" – Comparison
Tetanus is an acute infectious disease caused by Clostridium tetani. The severe consequences following its onset demand that prevention and treatment be race against time.
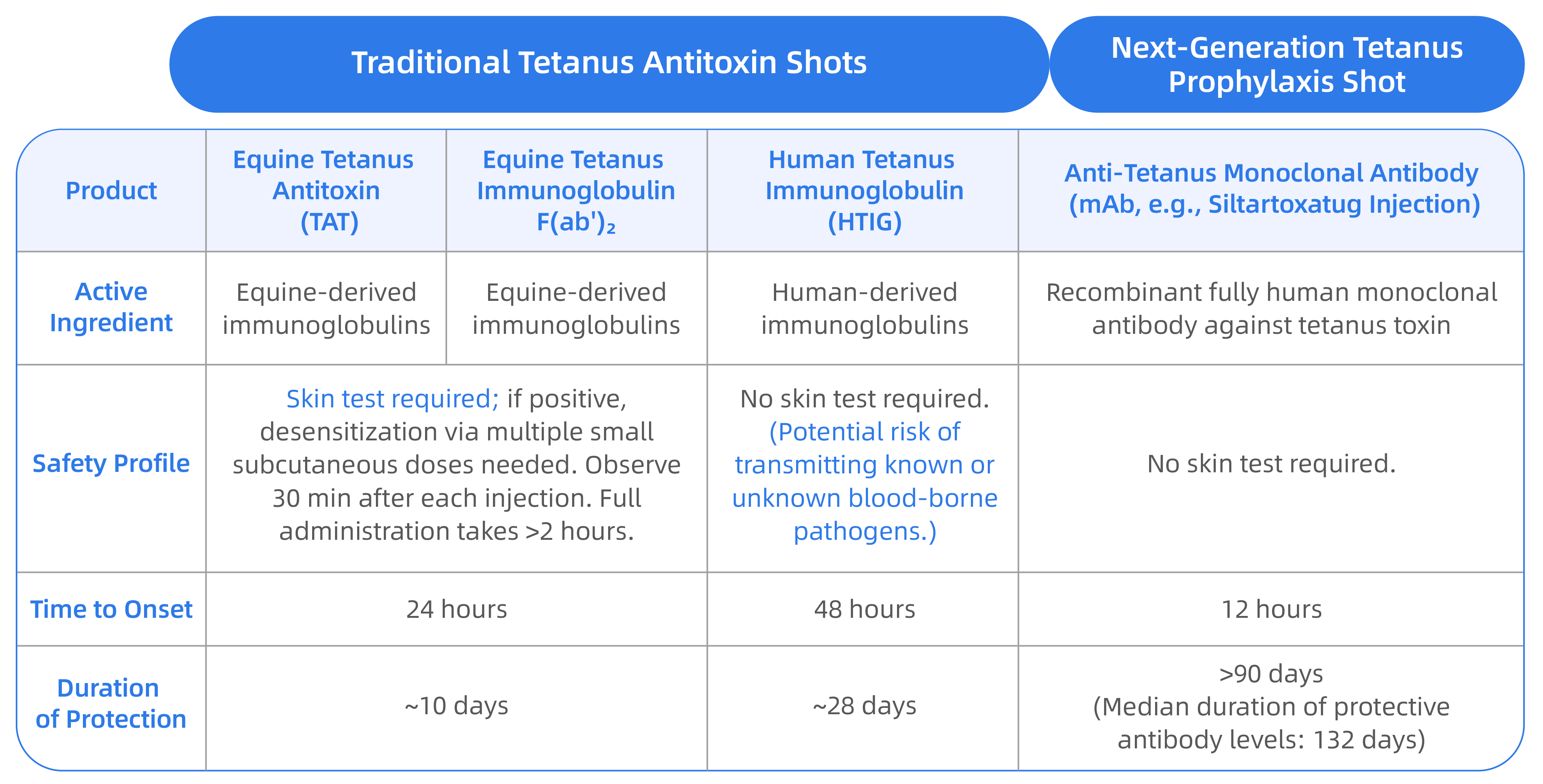
According to the Expert Consensus on the Application of Passive Immunization Agents for Post-Exposure Tetanus Prophylaxis, anti-tetanus monoclonal antibody (e.g., Siltartoxatug Injection) should be the preferred agent when passive immunization is indicated. This authoritative guidance provides clinicians with a clear rationale for medication selection, effectively promoting the standardization of tetanus prophylaxis practices. The consensus notes that anti-tetanus monoclonal antibodies have grater application value in cases of high-risk tetanus wounds, without boost tetanus vaccination over 10 years, or requiring simultaneous vaccination with rabies or other vaccine. Furthermore, for patients with severe trauma, head/face/neck injuries, wounds exposed for over 6 hours, immunocompromised status, or underlying conditions like diabetes, these monoclonal antibodies provide more enhanced protection and, and especially more effective for patients with either short (<2 or="" long="">30 days) incubation periods.
Tetanus is an acute infectious disease where prevention requires public awareness. Safety is paramount, and prophylaxis is key. In case of injury, especially deep or contaminated wounds, prompt medical evaluation for tetanus prophylaxis is essential. The anti-tetanus monoclonal antibody offers key advantages: faster onset of action, higher neutralizing antibody titers, and a longer duration of protection. It successfully addresses the long-standing reliance on human and equine plasma-derived products, marking a significant therapeutic advancement.
(Sintetol Trademark Registration Number: Class 5-74174546)
Statement
1. Trinomab does not promote the use of any drug outside its approved indications.
2. The medical information in this press release is intended for informational and news reporting purposes only and is not intended for promotional use, nor should it be considered as healthcare or diagnostic advice.
Forward-Looking Statements
This press release contains information that is current as of the date of release. It may include certain forward-looking statements regarding future events or the Company’s future performance. These statements are subject to risks and uncertainties. Words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” and similar expressions are intended to identify such forward-looking statements. Trinomab undertakes no obligation to update any forward-looking statements, whether as a result of new information, future events, or otherwise.